The Capillary Tube Method for Finding the Saturated Vapour Pressure of Water
A capillary tube closed at one end containing a short column of air trapped by a small amount of water as shown below.
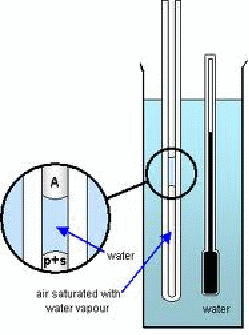
The trapped air is saturated with water vapour because of the short column of water. If atmospheric pressure is![]() and the pressure exerted by the trapped air is p, then, ignoring the pressure exerted by the short column of water is
and the pressure exerted by the trapped air is p, then, ignoring the pressure exerted by the short column of water is![]() so
so![]()
Saturated vapours do not obey the ideal gas law![]() Saturated vapour pressure is constant at a given temperature and independent of the volume of air.. We apply the ideal gas law to the enclosed air, obtaining
Saturated vapour pressure is constant at a given temperature and independent of the volume of air.. We apply the ideal gas law to the enclosed air, obtaining![]() where
where![]() is the volume of trapped air and
is the volume of trapped air and![]() is the temperature. The constant can be evaluated if the value of the saturated vapour pressure at some temperature is known, then s can be found for any other temperature
is the temperature. The constant can be evaluated if the value of the saturated vapour pressure at some temperature is known, then s can be found for any other temperature![]()