Graphite
Graphite has a giant molecular structure. The atoms are arranged in layers. Graphite has weak bonds between layers so the layers slip over each other making graphite soft and suitable to be used as pencil lead.
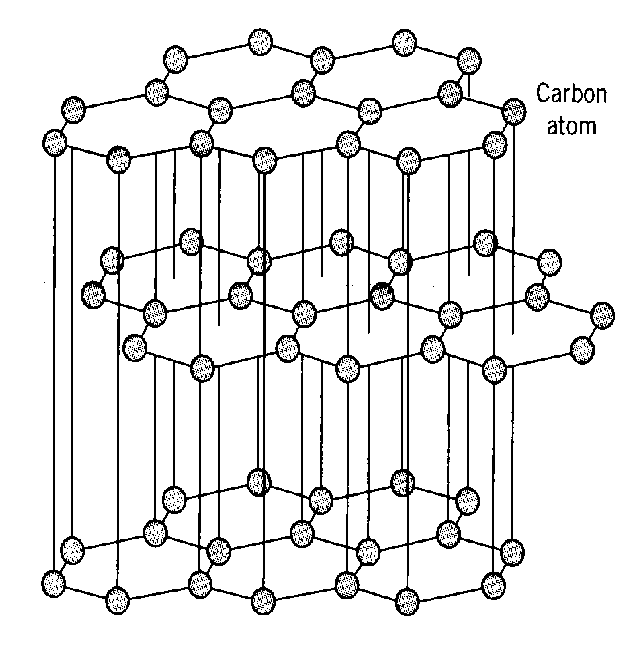
Graphite is not classified as a conductor. It does however conduct electricity but only within the layers of carbon atoms. Electrons find it much more difficult to conduct electricity from layer to layer and for this reason graphite is not classified as a conductor.
Each carbon atom is bonded to three other carbon atoms. The bonds are strong, and a lot of energy is needed to break them so the melting point of graphite is high.