Robert Millikan is credited with being the first person to measure the charge on an electron. He did this by balancing the electric and gravitational forces acting on a charged oil drop in a magnetic field. If the top plate is positively charged and the drop has an excess of electrons, the drop will be attracted to the upper plate. The voltage can be varied so that the gravtional force is just blanced by the electric force.
Using an atomiser he generated oil droplets above two parallel plates. The droplets fell through a small hole in the upper, isolated plate to the space between the plates and observed from one side through a microscope. The droplets acquired charge by exposing them to X – Rays, and thus experience an electric force when they entered the region between the plates.
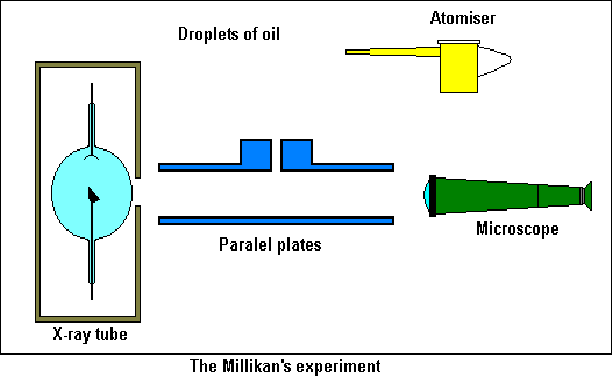
With no voltage applied, oil droplets we observed moving between the plates. Two forces acted – air resistance given by Stoke's Law,![]() and gravity
and gravity![]() When these balance the drop falls with a uniform velocity. Equating these two and solving for m gives:
When these balance the drop falls with a uniform velocity. Equating these two and solving for m gives:![]()
When the electric field is turned on and additional force![]() acts on the drop, where d is the distance between the plates and V is the applied voltage. Assuming the drop has an excess of electrons, and that the top plate is positively charged,
acts on the drop, where d is the distance between the plates and V is the applied voltage. Assuming the drop has an excess of electrons, and that the top plate is positively charged,
![]()
![]()
Many experiments were done and it was found that q was always a integral multiple of![]() hence this was deduced to be the charge on an electron.
hence this was deduced to be the charge on an electron.