To ask the question, 'what keeps the el;ectron together?' might seem a little bit silly. The electron is supposedly an elementary particle – it cannot be split into smaller particles. It has a single entity and has no internal structure.
We have then a puzzle. The electron has a charge of![]() This charge localised on a single elementary particle is associated with an energy. This energy is the energy needed to bring the charge to a point. Because the electric potential around a point charge is given by
This charge localised on a single elementary particle is associated with an energy. This energy is the energy needed to bring the charge to a point. Because the electric potential around a point charge is given by![]() as
as![]() tends to 0, as required for a point charge, the energy needed to assemble the electron is infinite.
tends to 0, as required for a point charge, the energy needed to assemble the electron is infinite.
The electron is known from experiment to have a size of less than![]() We can this of the electron as two half spheres, each carrying half the charge of the electron
We can this of the electron as two half spheres, each carrying half the charge of the electron
The energy needed to assemble the electron is then![]()
The repulsive force on each half of the electron due the their mutual electric repulsion at this distance is almost 60 N.
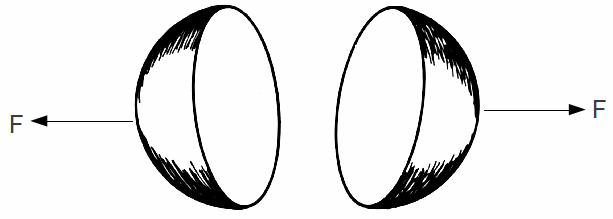
The strong nuclear force does not affect the electron. Obviously some other force is needed to hold the electron together. We do not know at the moment what this force could be.