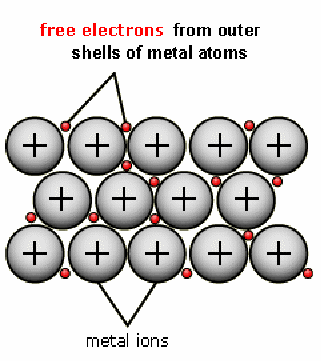
Metal is made up of a positively charged lattice of ions surrounded by electrons that are free to move. There free electrons are a product of the fact that metals easily lose electrons, being from the lower groups of the periodic table.
The electrons being free, if an electric field is applied, the electrons can move in the opposite direction to the field – since they are negatively charged.
The above explanation can be extended to explain why the resistance of a metal increases with increasing temperature. The atoms vibrate and impede the flow of electrons if the metal is heated, so the resistance of the metal to the flow of electrons – current – increases.