Alpha particles were fired at a high velocity into a very thin sheet of gold foil. The trajectories of these particles after passing through the foil were then detected. If the plum pudding model of the atom was correct, then it was assumed that many of the particles passing through would have had their courses slightly altered by the charge within the atoms, though in no dramatic fashion.
The result of the experiment differed remarkably from expectation. Where most of the alpha particles passed right through the gold atoms as if they weren’t even there, a small fraction of them got diverted at very dramatic angles. Some of the particles even bounced directly back in the opposite direction. Analysis of the results implied a size for the nucleus of around![]()
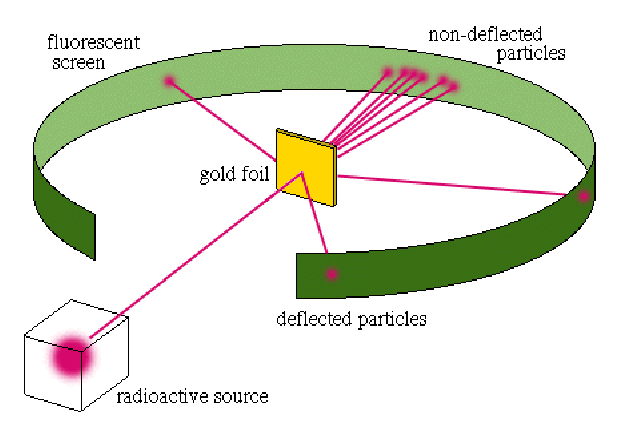
After analyzing the data, Rutherford realized that the plum pudding model could not account for this discrepancy, and in 1911 proposed a new and improved atomic model – called, appropriately, the Rutherford Model. In this model, the negative electrons orbited around a tiny, centralized, positively charged and incredibly dense central “nucleus.” This was the first of what can be referred to as “solar system” models, where the electrons orbit the nucleus like the planets orbit the sun. While it is known today that there are certainly limitations to how well this model genuinely reflects what is really happening within an atom, it has proved tremendously useful in explaining certain very fundamental features of the atomic structure, specifically the behaviors of electrons, spectroscopic analysis, and the formation of atomic bonds.