For an ideal gas the internal energy depends only on the temperature![]() where
where
![]() is the number of mols and
is the number of mols and![]() is the Universal Gas Constant,
is the Universal Gas Constant,![]()
When the temperature increases by a small amount![]() the corresponding change in internal energy is
the corresponding change in internal energy is![]()
The specific heat capacity of a substance is defined as![]() so for a gas with
so for a gas with![]() mols we see the heat capacity at constant volume is
mols we see the heat capacity at constant volume is![]() and the molar heat capacity at constant volume, labelled
and the molar heat capacity at constant volume, labelled![]() is
is![]()
In fact the above equation only holds for gases whose particles are single atoms. It is more accurate to say that the molar hear capacity for an ideal gas is![]() per degree of freedom. This allows us to generalise to gases made up of molecules of two or more atoms.
per degree of freedom. This allows us to generalise to gases made up of molecules of two or more atoms.
For a monotomic gas there are 3 degrees of freedom: up and down, forwards and backwards, left and right.
For a diatomic gas there are two extra, 'vibrational' degrees of freedom and two extra 'rotational' degrees of freedom, each contributing an extra![]() to the molar heat capacity at constant volume. For a diatomic gas,
to the molar heat capacity at constant volume. For a diatomic gas,![]()
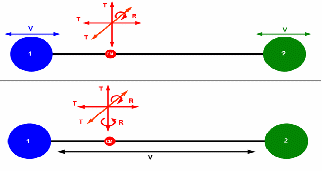
It must be noted though that the vibrational degrees of freedom only manifest above certain temperatures. For lower temperatures![]()