When an isolated - gas expands it cools. This demonstrates that between the gas molecules there exists an attractive force. When the gas expands, some of the kinetic energy of the gas molecules is used to overcome the attractive intermolecular forces between the gas molecules. The experimental apparatus is shown below.
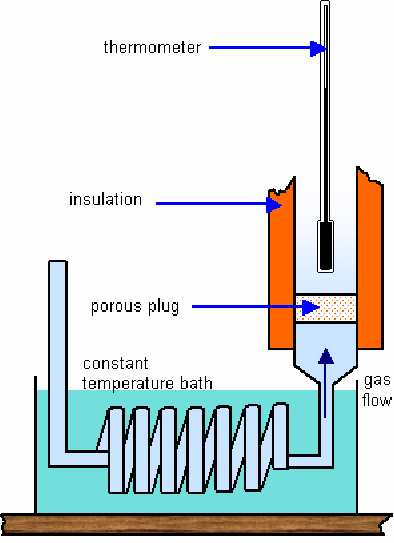
A high pressure gas is allowed to expand through a porous cotton plug designed to prevent any gain in kinetic energy of the gas as it passes through. The temperature of the gas will change on passing through the plug and the difference will be proportional to the pressure difference between the gases on either side of the plug – work is done on the gas in pushing it through the plug and by the gas in expanding when it emerges on the other side.
If the two pressures are the same, no work is done.
If the gas moves from high to low pressure, more work is done on the gas than by the gas. The gas cools. The table below shows typical temeprature changes per atmosphere difference in pressure.
|
Gas |
Temperature Change in Degrees Kelvin per atmosphere Pressure Difference |
|
Nitrogen |
-0.249 |
|
Oxygen |
-0.253 |
|
Air |
-0.208 |
|
Carbon Dioxide |
-1.005 |
|
Hydrogen |
+0.039 |