Alpha, Beta and Gamma Radiation
It is often said that there are three types of nuclear radiation: alpha, beta and gamma radiation respectively.
Alpha radiation consists of helium nuclei - two protons and two neutrons.
Beta radiation consists of fast electrons. When a nucleus undergoes beta decay, another particle called an anti – neutrino is also emitted. In alpha and beta decay the atom changes and becomes another element. When a nucleus undergoes gamma decay, the element does not change. The nucleus becomes less energetic.
Nuclear radiation is emitted when the nucleus undergoes some sort of decay. If it undergoes alpha or beta decay the atom changes and becomes another element.
Alpha Decay:![]()
Beta Decay:![]()
Gamma decay:![]()
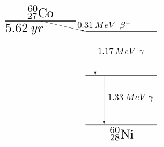
Sometimes an element may undergo a series of decays. A simple example is shown below. The![]() nucleus undergoes beta decay and two gamma decays. The half life of each decay is different.
nucleus undergoes beta decay and two gamma decays. The half life of each decay is different.
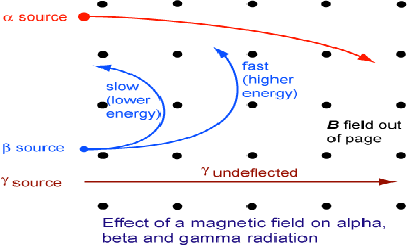
The three types of radiation can distinguished using magnetic fields. Gamma radiation is not charged and it will not be affected by the field and will pass through undeflected. Alpha radiation and beta radiation are oppositely charged and will bend in opposite directions as predicted by the left hand rule.
Radioactivity is dangerous and can cause radiation sickness and kill.
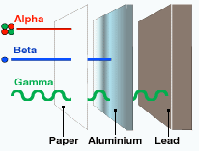
Alpha radiation can be stopped with a piece of paper or a few cm of air. However if a source of alpha radiation swallowed it is the most deadly because all the radiation is absorbed by the body. Beta radiation can be stopped with a few cm of aluminium and gamma radiation is the most penetrating and needs several cm of lead. If a source of gamma radiation is swallowed, most of the radiation will escape the body and do no harm.
Alpha radiation is used in smoke detectors, beta radiation is used to control the thickness of sheet metal and gamma rays are used to treat cancer and detect leaks in pipes.