How cold is it possible to get? Obviously not 0 C, since temperatures well below zero are common in the UK on winter mornings. In fact is is possible to reach very, very low temperatures indeed. We can estimate the lowest possible temperature by cooling gases. Ideal gases obey Charles' Law![]() where V = volume and T = temperature, if the pressure is kept constant. We can plot a graph of Volume against temperature for several different gases, obtaining a graph like that below.
where V = volume and T = temperature, if the pressure is kept constant. We can plot a graph of Volume against temperature for several different gases, obtaining a graph like that below.
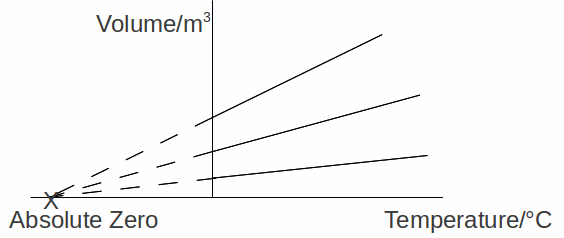
If we continue cooling the gases below absolute °C, the gas continues to contract, until, apparently they occupy no volume. This happens apparently at the same temperature whatever the gas. At this point, the molecules of the gases will have no kinetic energy and, we deduce cannot be cooled any further, so have a temperature of absolute zero (in fact, the gases will have condensed and solidified before this stage).
Measurements indicate that the absolute zero of temperature exists at – 270.16°C. Temperature is often given in °K (K=Kelvin). The Kelvin scale is called the absolute temperature scale and is such that 1°C =1 °K, It is important to note that Charles' Law![]() must be used with T in °K. If the temperature is given in °C, you can change it to °K by adding 273.16, though in fact this is usually approximated so that we only add 273.
must be used with T in °K. If the temperature is given in °C, you can change it to °K by adding 273.16, though in fact this is usually approximated so that we only add 273.