The evidence of energy levels in atoms is obvious from emission and absorption spectra. Each element has a set of frequencies of radiation which can be absorbed or emitted, corresponding to the energy levels between atoms.
An atom is illustrated below.
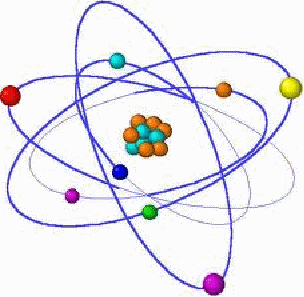
Electrons orbit the nucleus in essentially the same way as planets orbit the Sun. Bohr assumed that unlike planetary orbits, which can have a range of energies, the orbits of electrons are restricted by the angular momentum of the electrons. Bohr assumed the electrons in hydrogen had angular momentum![]() equal to an integral multiple of Planck's constant
equal to an integral multiple of Planck's constant![]() divided by
divided by![]() where
where![]() This gave rise to energy energy levels (
This gave rise to energy energy levels (![]() )in exact agreement with experiment.
)in exact agreement with experiment.
There were problems however. Why angular momentum should be quantized is not obvious – the model just works to predict the energy levels in the hydrogen atom accurately.
The model does not work for heavier atoms, only for hydrogen. Hydrogen has only one electron, but heavier atoms have more than one electrons interacting with each other.
The electron is a charged particle, and because it is moving around the nucleus, it should be constantly accelerating and hence emitting radiation. The electrons should thus be losing energy and should spiral into the nucleus. That they do not shows the model is incomplete, even for hydrogen.