The Second Law of Thermodynamics
The second law of thermodynamics can be stated in many ways. In principle there is nothing to stop the complete conversion of thermal energy into useful work. In practice though, a gas cannot continue to expand forever (thus reaching a temperature of absolute zero). The apparatus imposes limitations, the net result being that the continuous conversion of thermal energy into work requires a cyclical process – a heat engine. As shown below, a heat engine must lose some heat to the cold reservoir. This lost heat cannot be converted into work done.
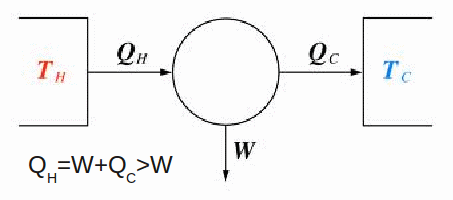
The first law of thermodynamics![]() implies
implies![]()
This can be expressed as the Kelvin – Planck formulation of the second law:
'No heat engine operating in a cycle, can convert heat energy from its' surroundings into work with 100% efficiency'.
Equivalently, 'a heat pump (not engine – a heat pump is a heat engine in reverse) may not transfer heat from a cold to a hot reservoir without work being done on it'.
More obviously, most people will know 'heat flows from hot to cold objects'.
Less obviously and more technically, 'the entropy of the universe can never decrease'.