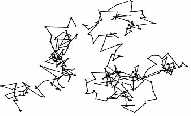Robert Brown, a British botanist, was observing through a microscope particles of pollen suspended in water. The smaller particles moved continuously in a 'random walk'. We now know this to be due to the bombardment of the pollen particles by the liquid molecules. The direction of the resultant force changes continuously, as more liquid molecules hit the pollen grain on one side or the other. Now 'Brownian motion' is defined as the random motion of suspended particles.
Experiment:
Brownian motion can be demonstrated by releasing smoke particles from burning cord into a glass container and putting a cover plate to seal the container.
To see brownian motion in a liquid place some water with graphite particles - pencil lead - suspended in it into a container.
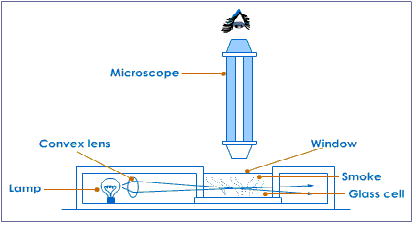
Adjust the microscope slightly until you can see graphite or smoke particles. The particles of scatter (reflect) the light shining on them and as points of light in random motion. The graphite (or smoke) particles are much larger than the water (or air) molecules, which are far to small to see even with a microscope.
The irregular movement of the particles is explained as being due to an uneven bombardment of the particles by the invisible molecules of water (or air). A possible irregular path of a smoke or graphite particle is shown..