In atoms, electrons are arranged in shells. Each shell can accommodate only up to a certain number of electrons:
The first shell can accommodate two electrons.
The second shell can accommodate up to eight electrons.
The third shell can accommodate up to eight electrons.
The number of electrons in a shell determines the group in which an atom lies. An atom with one electron in the outermost shell is in group 1. An atom with two electrons in the outermost shell is in group 2 and so on up to group 7. An atom with a full shell is in group 0. Atoms in this group have eight electrons in the outermost shell. The 0 can be taken to mean there are no electrons (yet) in the next shell.
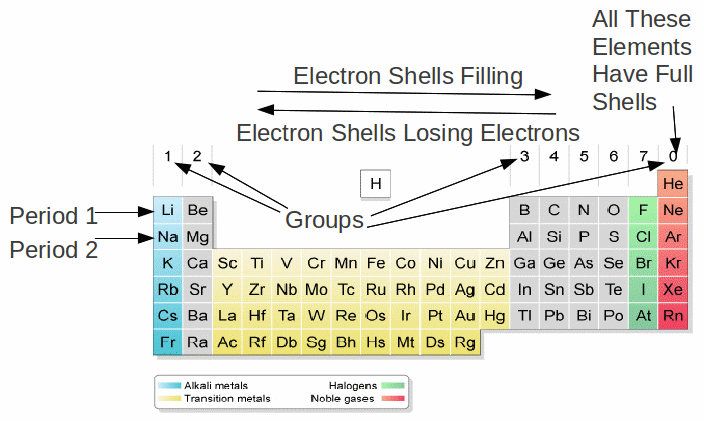
The number of electrons in a shell determines the number of chemical bonds an atom can form. Atoms feel the need to obtain full shells of electrons by either gaining electron from other atoms, moving closer to filling a partially empty shell, by losing electrons to other atoms, moving closer to losing all the electrons in that partially filled shell. Losing or gaining electrons is basically the same as forming a chemical bond.
Any atom with one electron in the outermost shell – group 1 in the periodic table – can form only one chemical bond, by losing one electron.
Any atom with two electrons in the outermost shell – group 2 in the periodic table – can form up to two chemical bonds, by losing two electrons.
This continues for periods 1 and 2 – the rows in the body of the table – up to the fourth group, carbon and silicon, which have four electrons in the outermost shell and can form up to four chemical bonds. These atoms act as electron donors or sharers – they donate atoms to elements such as fluorine, which need electrons to make full electron shells, or share electrons to form covalent bonds with elements such as hydrogen.
Any atom with eight electrons in the outermost shell – group 0 in the periodic table – cannot form chemical bonds, since these atoms have full shells.
Any atom with seven electrons in the outermost shell – group 7 in the periodic table – can form only one chemical bond, by gaining an electron and obtaining a full shell.
Any atom with six electrons in the outermost shell – group 6 in the periodic table – can form only two chemical bonds, by gaining two electrons and obtaining a full shell.
Because the number of electrons in the outermost shell determines which group an element is in and how many chemical bonds it can form, elements with similar chemical properties lie in the same group. This is the fundamental meaning of the periodic table.