Atoms consist of a nucleus consisting of positively charged protons and neutral neutrons, surrounding by electrons moving in predefined orbits or shell.. The protons and neutrons together form the nucleons. Each nucleon has almost the same mass, and the mass of a nucleon is a convenient unit, so the mass of a nucleus consisting of 2 neutrons and 1 proton is taken to be 3.
The electron is very nearly massless, having only 1/1800 the mass of a nucleon.
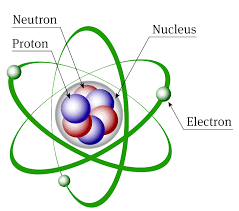
Protons and electrons are oppositely charged, but the charges are the same size. Atoms contain equal number of protons and neutrons, so the atom as a whole is neutral. There are usually more neutrons in the nucleus than protons. The protons are positive and repel each other, and the nucleus is only stable because of the presence of neutrons and the always attractive 'strong force' between nucleons in the nucleus.

