JJ Thomson
JJ Thomson discovered the electron and determined it's charge to mass ratio![]()
Experiment showed that if the cathode rays were made to hit a gold leaf electroscope, the leaf would deflect. Investigation implied the cathode rays carried a negative electric charge. This implied they would be deflected by an electric field, but experiment showed they were undeflected.
JJ Thomson realised thought there might be some mechanism that caused the gas to act as a conductor. If the cathode rays move inside a conductor, they would not be deflected by an electric field. By removing as much of the gas as possible he did obtain a deflection for the cathode rays in an electric field, providing strong evidence for the cathode rays being negatively charged.
To measure the charge to mass ratio he used the arrangement below:
<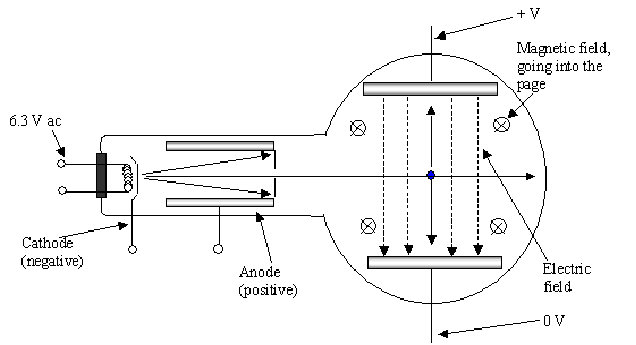
The magnetic and electric fields produce forces to deflect the the cathode rays in opposite directions. The idea is to balance the electric and magnetic forces on the cathode rays so there is no overall deflection.
After leaving the cathode the electrons are accelerated though a voltage![]() gaining kinetic energy
gaining kinetic energy![]() where
where![]() = charge of the particles and
= charge of the particles and![]() is their mass. Rearrangement gives
is their mass. Rearrangement gives![]() (1)
(1)
Magnetic force on a charged particle = electric force
![]() (2)
(2)
![]() = magnetic field strength
= magnetic field strength
![]() = speed of charge carriers
= speed of charge carriers
![]() = electric field strength =
= electric field strength =![]() where
where![]() is the applied voltage between the plates and
is the applied voltage between the plates and![]() is the plate separation.
is the plate separation.
Substitution of (1) into (2) gives![]()
All the quantities on the right hand side can be measured and![]() found.
found.