Ideal Gas Processes
An ideal gas can undergo an number of changes, but there are four very important types of change, each obeying the first law of thermodynamics (![]() ). We can represent each type of change on a pV diagram.
). We can represent each type of change on a pV diagram.
Isochoric or Isovolumetric#. The gas has a constant volume.
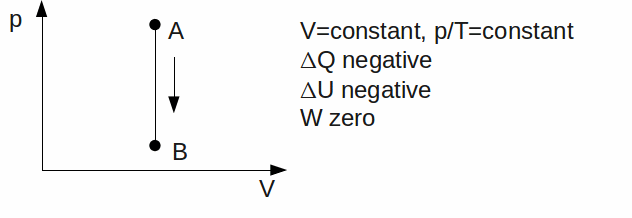
Isobaric. The gas has a constant pressure.
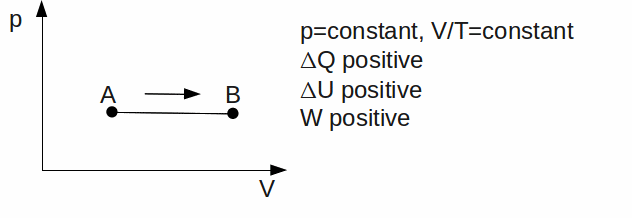
Isothermal. The temperature is kept constant. Since U is a function of temperature only,
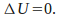
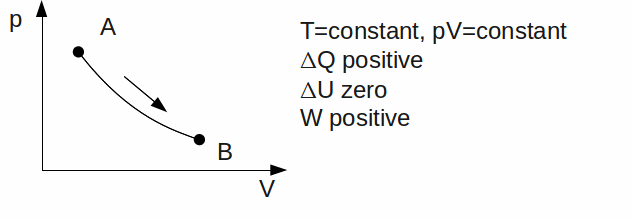
Adiabatic. No heat exchange takes place between the gas and its' surroundings. Any work done by the gas therefore means a decrease in it's thermal energy. Rapid expansions or compressions are approximately adiabatic, because these allow little time for heat transfer.
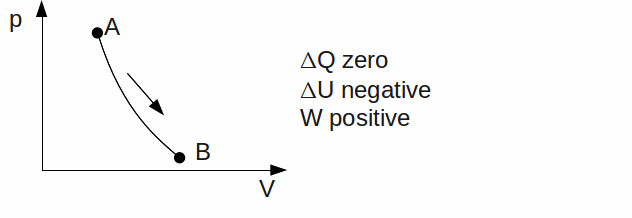
If the direction of any of the above processes is reversed, the signs of![]() and
and![]() will change.
will change.