Capillary Action
The force of attraction between similar types of molecules is called cohesion. Cohesive forces are responsible for surface tension in liquids. There is also an attractive force between different types of molecules. This force is called adhesion – the name adhesive, commonly used for glues is derived from this.. Adhesive tape sticks to the surface of the skin because of the adhesion between the molecules of the tape and those of the skin. The curved surface of water in a test tube or glass is caused by adhesive forces between water molecules and the atoms and molecules in the glass. The water molecules are made to crawl up the side of the glass slightly because of this force of adhesion.
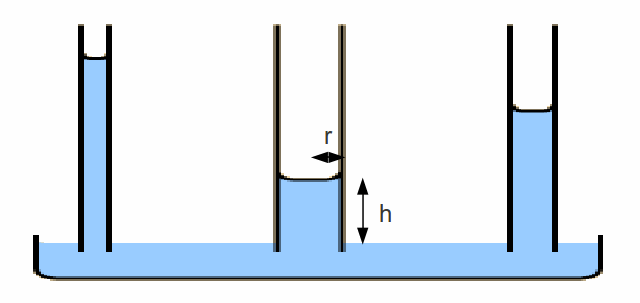
The thinner the glass tube, the higher the water will crawl.
Consider the middle tube. At a height![]() the water exerts a
the water exerts a![]() and to get to this height there must be a force between the water and glass of magnitude
and to get to this height there must be a force between the water and glass of magnitude![]() where
where![]() is the surface tension between the water and glass. Setting these two equal gives
is the surface tension between the water and glass. Setting these two equal gives
![]()
This equation explains the diagram – the water climbs higher with decreasing radius.
Nature uses capillary action in a great many places. Sap uses capillary action to circulate through trees and plants.