Allotropes
Different materials made up of the same element may have different properties because the material may have a different structure, EVEN THOUGH THEY ARE MADE UP OF THE SAME ELEMENT (do not confuse this with the phase of material – whether it is liquid, solid or gas). The reason is that atoms – even the same atoms - may combine in different ways to form materials with different structures.
Probably the most common allotropes, some of which many people may know, are allotrope of carbon. Carbon forms diamond, used in jewellery and industry and graphite, used in pencil lead. Though both of these materials are made of the same element, their physical properties are very different. Diamond is transparent and graphite is black. Diamond is hard and graphite is soft. Diamond is an electrical insulator and graphote partially conducts, depending on it's orientation. There are in fact many allotropes of carbon, eight so far discovered– amorphous (coal), nanofoam, lonsdalelite, ceraphite, buckyballs, nanotubes and graphene, discovered recently.
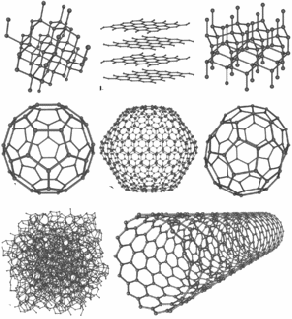
New allotropes for carbon have also been proposed.
Other elements also have allotropes -oxygen (as![]() and
and![]() or ozone), sulphur, tin and phosphorous among them. Generally one allotrope will be far more abundant than another. For example, the diamond allotrope of carbon is far less common than graphite. Phosphorous comes in at least 3 allotropic forms; red, black (or purple, or violet), white (or yellow). Red and white phosphorous are the most common, both of which consist of tetrahedrally arranged groups of four phosphorous atoms. The tetrahedral arrangements in red phosphorous are linked into chains, whereas those in white phosphorous are separate. Black phosphorous is arranged in 2-dimensional hexagonal sheets like graphite. White prosphorous reacts immediately to the air, oxidising and producing phosphorous pentoxide. American and Israelis like to bomb people with white phosphorous bombs.
or ozone), sulphur, tin and phosphorous among them. Generally one allotrope will be far more abundant than another. For example, the diamond allotrope of carbon is far less common than graphite. Phosphorous comes in at least 3 allotropic forms; red, black (or purple, or violet), white (or yellow). Red and white phosphorous are the most common, both of which consist of tetrahedrally arranged groups of four phosphorous atoms. The tetrahedral arrangements in red phosphorous are linked into chains, whereas those in white phosphorous are separate. Black phosphorous is arranged in 2-dimensional hexagonal sheets like graphite. White prosphorous reacts immediately to the air, oxidising and producing phosphorous pentoxide. American and Israelis like to bomb people with white phosphorous bombs.