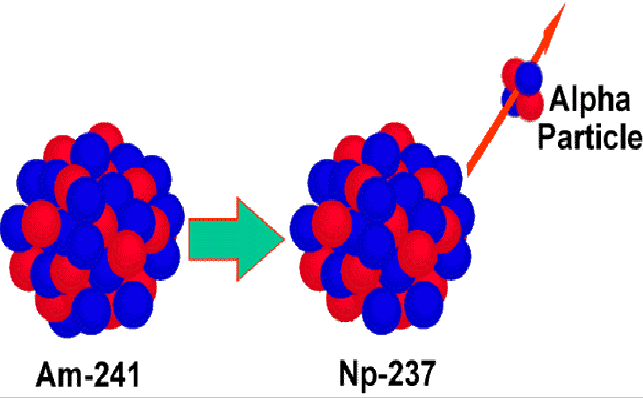
Alpha decay occurs when an atomic nucleus emits an alpha particle (a helium nucleus,![]() consisting of 2 protons and two neutrons) and decays into an atom with a mass and atomic numbers 4 and 2 less than the parent atom. For example
consisting of 2 protons and two neutrons) and decays into an atom with a mass and atomic numbers 4 and 2 less than the parent atom. For example
![]() shown below.
shown below.
Alpha decay is a quantum tunnelling process. The alpha particle has insufficient energy to escape the nucleus according to classical mechanics, but quantum mechanical effects mean it has a non zero chance of escaping the nucleus.
Alpha decay occurs in the heaviest nuclides. Theory implies it can occur only in nuclei heavier than nickel (element 28), beyond which binding energy per nucleon is increasing, so energy may be released by reducing the number of nucleons, and the nuclides are therefore unstable toward spontaneous fission-type processes.
Alpha particles have a typical kinetic energy of 5 MeV (that is,![]() of their total energy) and a speed of
of their total energy) and a speed of![]()
Because of their relatively large mass, +2 electric charge and relatively low velocity, alpha particles are very likely to interact with other atoms and lose their energy, so their forward motion is effectively stopped within a few cm of air. In cloud chambers they produce thick tracks, and in the human body they are the most dangerous form of radiation.
Most of the helium produced on Earth (approximately 99% of it) is the result of the alpha decay of underground deposits of minerals containing uranium or thorium. The helium is brought to the surface as a byproduct of natural gas production.