Desalination
Water containing more than 0.1 per cent salt by number of atoms is generally considered saline water. Typical sea water is about 3.5 % salt. Desalination is the removal of salt from water to make the water drinkable.
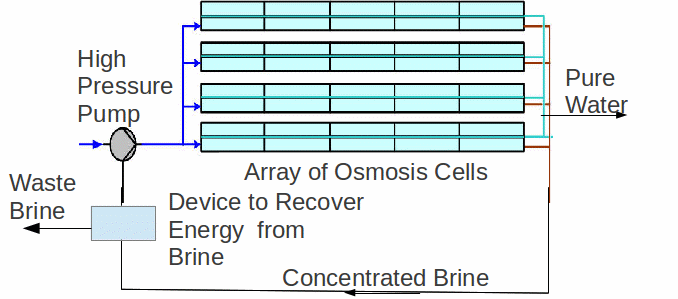
Currently the most common method of desalination is osmosis. Pressure is used to push the water solution through a membrane, with the membrane preventing the larger solutes/ions from passing. Osmosis is generally considered to be the least energy consuming of all the large-scale processes. The apparatus is shown below.
On ship, another method is possible because of large amounts of waste heat energy produced by the ship's engines. Seawater can be boiled, then condensed to give pure water, though in practice a small amount of a salt mixture is added back to make it more like naturally occurring water. It is not necessary to heat the water to boiling point. The water can be made to boil at a lower temperature by reducing the pressure using a vacuum pump.