Evaporation
Liquids evaporate when molecules in the liquid leave. A molecule must have a certain amount of energy to leave the liquid, because all the molecules of the liquid attract each other, so to escape the liquid a molecule must have sufficient energy to overcome these attractive forces. Molecules may and do reenter the liquid (condensation), so for net evaporation to take place more molecules must leave than reenter the liquid. This is shown below for water.
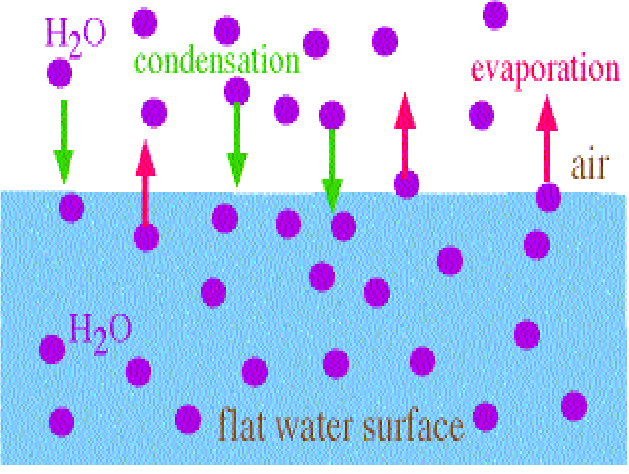
The rate of evaporation may be increased by
Increasing the temperature. This means moleculesmove faster and have more kinetic energy, so are more likely to beable to overcome the force attracting it to the other molecules inthe liquid.
Decreasing the air pressure. It is a well knownfact that decreasing the air pressure mean that liquids boil at alower temperature. A lower air pressure means that molecules cantravel further before coming into contact with other molecules,soare more likely to be able to escape the vicinity of the liquid oncein the air.
Letting air flow over the surface of the liquid.The air molecules that have escaped the liquid may be carried awayfrom the liquid surce by the flow of air, so condensation isreduced, and net evaporation is increased.