Solids, Liquids and Gases
At the macroscopic level, the level at which you directly observe with your senses, a solid has a definite shape and occupies a definite volume. An ice cube is a solid. You can easily weigh the ice cube and measure its volume. At the microscopic level (where items are so small that people can't directly observe them), the particles that make up the ice are very close together and aren't moving around very much ((below bottom left). In a solids, the particles are pulled into a rigid, organized structure of repeating patterns called a crystal lattice. The particles that are contained in the crystal lattice are still moving, but barely — it's more of a slight vibration. Depending on the particles, this crystal lattice may be of different shapes.
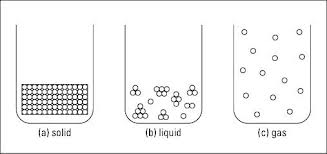
Liquid
When an ice cube melts, the bonds between the molecules break and the ice cube becomes a liquid. Unlike solids, liquids have no definite shape, but they do have a definite volume as liquids are virtually incompressible. For example, a cup of water in a tall skinny glass has a different shape than a cup of water in a pie pan, but in both cases, the volume of water is the same — one cup. The particles in liquids are farther apart than the particles in solids, and moving around much more - see the miccle diagram above. Even though the particles are farther apart in liquids than in solids, some particles in liquids may still be near each other, clumped together in small groups. Because the particles are farther apart in liquids, the attractive forces among them aren't as strong as they are in solids — which is why liquids don't have a definite shape. However, these attractive forces are strong enough to keep the substance confined in one large mass — a liquid.
Gases
If you heat water, you can convert it to steam, the gaseous form of water. A gas has no definite shape and no definite volume. In a gas, particles are much farther apart than they are in solids or liquids – top right diagram above - and they're moving relatively independent of each other. Because of the distance between the particles and the independent motion of each of them, the gas expands to fill the area that contains it (and thus it has no definite shape).