Air is made up of atoms and molecules. Atoms and molecules has mass, and so does air. Air is not some weightless substance that pervades space. It is just not very dense: the term 'lighter than air' is significant here. Things may float in air if they are less dense, but the air itself still has mass and weight. The density of air may be measured using the apparatus below.
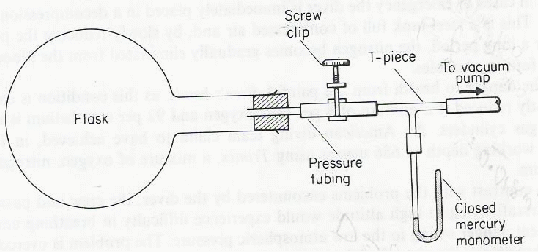
A clean round bottomed flask is fitted with a rubber bung. A glass tube passes through the bung and is connected to a vacuum pump via a screw clip.
With the clip open air is pumped out of the flask. All the air is gone when the levels are the same on both sides of the closed mercury thermometer. The clip is closed and the mass![]() of the flask measured.
of the flask measured.
The flask is connected to a calcium chloride drying tube and the clip is opened. Dry air enters the flask. The mass![]() is measured. The volume of the flask
is measured. The volume of the flask![]() can be found by filling it with water through the open clip, and pouring the water into a measuring beaker.
can be found by filling it with water through the open clip, and pouring the water into a measuring beaker.
![]()
The density of air under normal conditions is about![]() or
or![]() about 1 800 th of the density of water.
about 1 800 th of the density of water.