The most common type of battery used today is the "dry cell" battery. There are many different types of batteries ranging from the relatively large flashlight batteries to the minaturized versions used for wristwatches or calculators. Although they vary widely in composition and form, they all work on the sample principle. A dry-cell battery is essentially comprised of a metal electrode or graphite rod (elemental carbon) surrounded by a moist electrolyte paste enclosed in a metal cylinder as shown below.
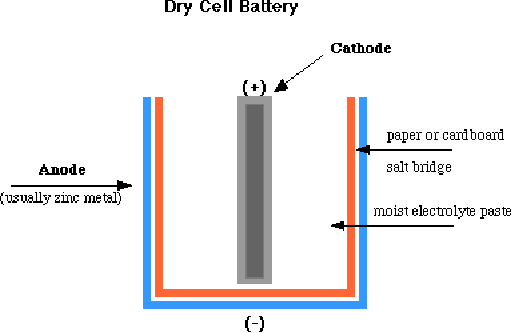
In the most common type of dry cell battery, the cathode is composed of graphite and the anode is composed of zinc. In an acidic dry cell, the reduction reaction occurs within the moist paste comprised of ammonium chloride (NH 4 Cl) and manganese dioxide (MnO 2 )
This dry cell produces about 1.5 volts, though a higher voltage can be produced by linking batteries in series. In the alkaline battery the ammonium chloride is replaced by KOH or NaOH.
Other types of dry cell batteries are the silver battery in which silver metal serves as a cathode to support the reduction of silver oxide (Ag2O) and the oxidation of zinc (anode) in a basic medium. The type of battery commonly used for calculators is the mercury cell. In this type of battery, HgO serves as the oxidizing agent (cathode) in a basic medium, while zinc metal serves as the anode. Another type of battery is the nickel/cadmium battery, in which cadmium metal serves as the anode and nickel oxide serves as the cathode in an alkaline medium. Unlike the other types of dry cells described above, the nickel/cadmium cell can be recharged like the lead-acid battery.