The Mechanism of Light Production by a Gas Discharge Tube
Gas is normally not a good conductor of electricity. The molecules of the gas are electrically neutral and the gas contains very few ions. This means that in order for a gas to conduct electricity, a source of electrons is needed. In a gas discharge tube the source of electrons is a negatively charged electrode (the cathode), relative to which the positive electrode (the anode) is at a high voltage. The electrons – also called cathode rays here because they originate from the cathode - are emitted from the cathode and accelerated towards the positively charged anode.
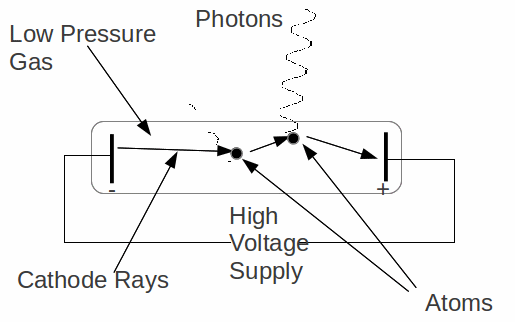
As they accelerate towards the anode, the electrons may collide with gas atoms. In doing so they may raise electrons in the gas atoms to higher energy levels. The electrons of the cathode rays are deflected, but continue to be accelerated towards the positively charged anode. Meanwhile, the electrons that are raised to a high energy level will quickly return to their original, lower energy level, emitting photons (light particles) as they do so. The frequency of the emitted light, hence the colour is characteristic of the gas in the discharge tube. Every gas emits light of different colours.
Discharge tubes are widely used as advertising signs. Neon is especially popular, are produces red light.