Suppose that some liquid is poured into a bottle which is then corked up. Because of evaporation, the space above the liquid begins to fill with vapour. The molecules in the vapour move about in all directions and exert a pressure when they bounce off the walls of the bottle. The also strike the surface of the liquid and may re-enter it. Eventually a state of dynamic equilibrium is reached in which the rate at which molecules leave the liquid is equal to the rate at which others return to it. The use of the word dynamic to describe the equilibrium stresses the fact that the molecules are in continuous motion with equal two way traffic at the surface of the liquid.
Under these conditions the space above the liquid is said to be saturated with vapour, and the pressure exerted is called the saturated vapour pressure.
At a given temperature the saturated vapour pressure is always the same
-
whether there is air in the space above the liquid or not.
-
Whatever the volume of the space above the liquid, as long asthere is still liquid present.
If the temperature increases then the molecules of the liquid and vapour move around faster. The molecules of the liquid are easier able to escape the attractive forces in the liquid because of their higher energy. More molecules will enter the vapour and the saturated vapour pressure will increase: saturated vapour pressure increases with temperature.
Measurement of Saturated Vapour Pressure
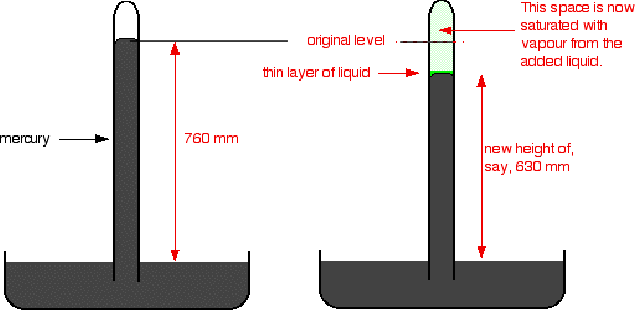
In the diagram below a small amount of liquid introduced at the top of the mercury column results in the column dropping from 760 mm to 630 mm. The saturated vapour pressure of the liquid is 130 mm.