Made of gallium arsenide or gallium phosphide, the forward biased light emitting diode, symbol shown below,
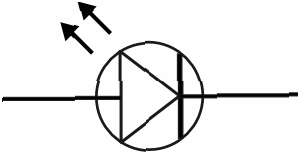
emits light when a voltage is applied, due to recombination of holes at the p – n junction. The p – n junction is very close to the surface. The colour of the emitted light depends on the exact composition of the diode.
The light – emitting diode is more more efficient than other devices – such as incandescent/filament bulbs, using maybe one quarter of the energy – that produce light and are widely used in electronics as indicator lights. Torches made using light emitting diode last much longer than traditional torches. Being a diode, they will begin to emit light as soon as the light reaches a certain voltage, so a user can be quite sure if a problem is indicated.
LEDs are available in all colours but blue and white LEDs are much more expensive than those producing other colours. LED's can be made that produce more than one colour. The tri-colour LED has a red and a green LED combined in one package with three leads. They are called tri-colour because mixed red and green light makes yellow and, produced when both the red and green LEDs are on.
Bi-colour LEDs
A bi-colour LED has two LEDs wired in inversely in parallel combined in one package with two leads. Only one of the LEDs can be lit at one time and they are less useful than the tri-colour LEDs described above.
