A general atom is shown above. The nucleus is made up of blue and yellow dots at the centre and the electrons are arranged in shells or rings around the centre. The rings represent energy levels, with the energies of the electrons increasing as we move out from the centre. We may also illustrate the energy levels on a ladder diagram, shown below for hydrogen.
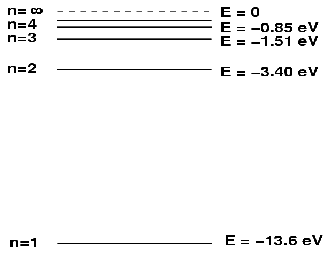
When a photon enters the atoms, it may for example, hit the electron in the![]() shell and if that photon has energy at least equal to the difference between the
shell and if that photon has energy at least equal to the difference between the![]() energy level, -3.4eV, and the
energy level, -3.4eV, and the![]() energy level -13.6eV ie the photon energy is at least 10.2 eV, the electron may make a transition from the
energy level -13.6eV ie the photon energy is at least 10.2 eV, the electron may make a transition from the![]() level to the
level to the![]() level. The electron may than decay back down to the n=1 energy level with the emission of a photon of energy 10.2eV. The frequency of the emitted electron will be given by
level. The electron may than decay back down to the n=1 energy level with the emission of a photon of energy 10.2eV. The frequency of the emitted electron will be given by![]()
