Molecules in a liquid move at speed and undergo frequent collisions. A molecule follows a random, zigzag path, its course changing abruptly at each collision. A molecule might only move a fraction of a millimetre in one minute even though it's average speed is hundreds of metres per second. During the minute it might experience![]() collisions, each collision causing the speed and direction of motion to change.
collisions, each collision causing the speed and direction of motion to change.
If a group of another sort of molecules enter the liquid at one location, they also experience this random motion. As time progresses, the molecules move or diffuse away from the point at which they enter the liquid into the surrounding liquid. Diffusion is the slow net movement due to random collisions of atoms and molecules away from areas in which they are concentrated into areas in which they are less concentrated.
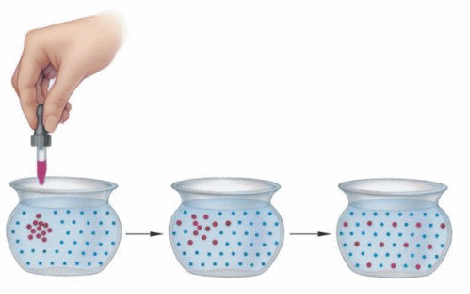
Diffusion occurs in all fluids, gases as well as liquids. It occurs faster in gases because the speed of the molecules is faster. It plays an important part in many biological processes. Oxygen released in capillaries diffuses from blood to cells near the capillaries, and muscles receive oxygen that diffuses from the surrounding material. Since muscles need oxygen to move, the rate at which muscles can do work is limited by the rate of diffusion of oxygen into muscle tissue.
