Osmosis is a form of diffusion that occurs across a membrane. The membrane allows small molecules to pass through but inhibits the passing of larger molecules. The skin of a wrinkled prune will allow water to pass though but not the larger sugar molecules inside the prune. When the prune is placed in water it absorbs water until it becomes swollen, not allowing sugar molecules to pass from inside the prune to outside. In the same way, a red blood cell placed in water swells because water passes through the cell membrane from outside to inside, but does not allow the larger haemoglobin molecules to pass from inside the cell to outside. In all cases of osmosis, there is a net flow of fluid to the region with the greatest concentration of dissolved molecules or ions.
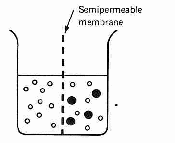
In the diagram above, on the left of the membrane is only water and on the right is a mixture of water and glucose molecules. The two types of molecules act much like molecules of gas. The sugar molecules create a pressure on the membrane we would expect from a group of atoms of gas hitting a wall:![]()
Next consider the pressure exerted by the water molecules. More water molecules per unit volume are on the left than the right hand side, so water molecules will hit the left hand side of the membrane at a greater rate than the right hand side, so there will be a net passage of water from the left to the right hand side. The fluid level on the left will fall and the level on the right will rise.
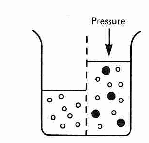
The concentration of sugar molecules is![]() so
so
