Uranium is a common element on Earth. It's been around since the planet formed around 4.6 billion years ago since its half life – for U238 - of 4.5 billion years is about the same. U-238 makes up 99 percent of the uranium on Earth, while uranium-235 (U-235) makes up about 0.7 percent of the uranium found naturally.
Uranium-235 has an interesting property that makes it handy for the production of both nuclear power and nuclear bombs. U-235 decays naturally, just as U-238 does, by emission of alpha particles: it emits an alpha particle, two neutrons and two protons bound together. U-235 also undergoes spontaneous fission a small percentage of the time. However, U-235 is one of the few materials that can undergo induced fission. If a free neutron runs into a U-235 nucleus, the nucleus will absorb the neutron, become unstable and split immediately.
The probability of a U-235 atom capturing a neutron as it passes by is high. In fact, under reactor conditions, one neutron ejected from each fission causes another fission to occur, shown below.
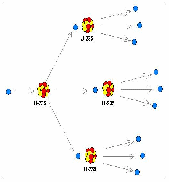
As soon as the nucleus captures the neutron, it splits into two lighter atoms and throws off two or three new neutrons (the number of ejected neutrons depends on how the U-235 atom splits). The process of capturing the neutron and splitting happens very quickly, on the order of picoseconds![]()
The decay of a single U-235 atom releases approximately 200 MeV (million electron volts). That may not seem like much, but there are a lot of uranium atoms in a pound (0.45 kg) of uranium. So many, in fact, that a pound of highly enriched uranium has enough can release as much energy as about a million gallons of gasoline.
The splitting of an atom releases an incredible amount of heat and gamma radiation, or radiation made of high-energy photons. The two atoms that result from the fission later release beta radiation(super fast electrons) and gamma radiation of their own as well. The energy released by a single fission comes from the fact that the fission products and the neutrons, together, weigh less than the original U-235 atom. The difference in weight is converted directly to energy at a rate governed by the equation![]()
However, for all of this to work, a sample of uranium must be enriched so that it contains more U-235. Three percent enrichment is sufficient for nuclear power plants, but weapons-grade uranium is composed of at least ninety percent U-235.
