The neutron is an uncharged particle with a mass –![]() - slightly larger than that of a proton.
- slightly larger than that of a proton.
While bound neutrons in stable nuclei are stable, free neutrons are unstable; they undergo beta decay with a mean lifetime of just under 15 minutes (885.7±0.8s). Free neutrons are produced in nuclear fission and fusion and are the key to nuclear power production. After the neutron was discovered in 1932, it was realized in 1933 that it might mediate a nuclear chain reaction. In the 1930's, neutrons were used to produce many different types of nuclear transmutations. When nuclear fission was discovered in 1938, it was soon realized that this might be the mechanism to produce the neutrons for the chain reaction, if the process also produced neutrons, and this was proven in 1939, making the path to nuclear power production evident. These events and findings led directly to the first nuclear chain reaction which was self-sustaining (1942) and to the first nuclear weapons in 1945.
Under the Standard Model of particle physics, because the neutron consists of three quarks, the only possible decay mode without a change of baryon number is for one of the quarks to change flavour via the weak interaction. The neutron consists of two down quarks with charge −1⁄3 e and one up quark with charge +2⁄3 e, and the decay of one of the down quarks into a lighter up quark can be achieved by the emission of a W boson. By this means the neutron decays into a proton (which contains one down and two up quarks), an electron, and an electron antineutrino.
Outside the nucleus, free neutrons are unstable and have a mean lifetime of 885.7±0.8 s (about 14 minutes, 46 seconds); Free neutrons decay by emission of an electron and an electron antineutrino to become a proton, a process known as beta decay:
Neutrons in unstable nuclei can also decay in this manner. However, inside a nucleus, protons can also transform into a neutron. This transformation occurs by emission of a positron and a neutrino or electron capture:
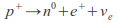 or
or
When bound inside of a nucleus, the instability of a single neutron to beta decay is balanced against the instability that would be acquired by the nucleus as a whole if an additional proton were to participate in repulsive interactions with the other protons that are already present in the nucleus. As such, although free neutrons are unstable, bound neutrons are not necessarily so. The same reasoning explains why protons, which are stable in empty space, may transform into neutrons when bound inside of a nucleus.
