A hot air balloon with a volume of 2,000 m3 full of air with a density of 1.29 kg/m 3 has a mass of 2,000*1.29 =2580 kg.
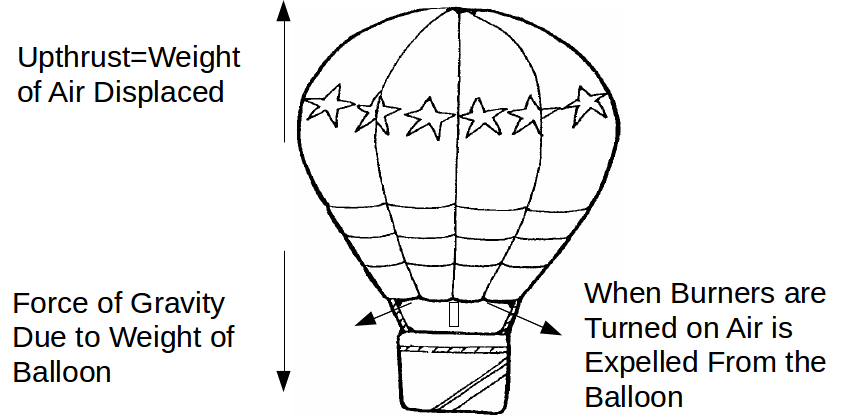
When the burner is turned on, the air inside the balloon is heated and starts to expand. Suppose it is initially at a temperature of 15 ° C and is heated to a temperature of 45 ° C. If the volume of the balloon does not change, then some of the air must escape the balloon – the balloon is open at the bottom, above the canopy.
We can use the ideal gas equation in the form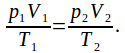
The pressure before and after the air is heated is atmospheric pressure, so![]() We can cancel the pressure from both sides to obtain
We can cancel the pressure from both sides to obtain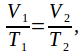 hence
hence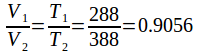 so
so![]() of the air in the balloon has escaped and the total mass of the balloon is now 2580 – 0.0944 times 2580=2367 kg. This decrease in mass of the balloon means an decrease in the force of gravity, but the buoyancy of the balloon has not changed, since this only depends on the volume of the balloon. The result may be enough to cause the balloon to start to rise.
of the air in the balloon has escaped and the total mass of the balloon is now 2580 – 0.0944 times 2580=2367 kg. This decrease in mass of the balloon means an decrease in the force of gravity, but the buoyancy of the balloon has not changed, since this only depends on the volume of the balloon. The result may be enough to cause the balloon to start to rise.
