Thermodynamic System – Most of the time when studying the bahaviour of an ideal gas in a particular situation, we focus on the macroscopic behaviour of the gas as a whole. The gas can gain or lose thermal energy, and either do work or have work done on it. Because of this, the gas can be considered a thermodynamic system.
The Surroundings – No system is completely isolated. To some extent, every system interacts with its' surroundings, exchanging heat, doing work or having work done by the surroundings.
Heat![]() – Refers to the exchange of heat energy between a system and its' surroundings as a result of a temperature difference – heat flows from hotter to colder bodies until the temperatures are the same.
– Refers to the exchange of heat energy between a system and its' surroundings as a result of a temperature difference – heat flows from hotter to colder bodies until the temperatures are the same.
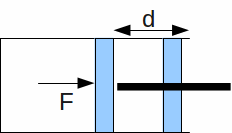
Work![]() – Refers to the macroscopic transfer of energy. For example, Work is done by a force
– Refers to the macroscopic transfer of energy. For example, Work is done by a force![]() if it forces a piston back a distance
if it forces a piston back a distance![]() Such a force could be exerted by a gas – when the gas expands it does work on its' surroundings. Conversely when the gas is compressed, the surroundings do work on the gas.
Such a force could be exerted by a gas – when the gas expands it does work on its' surroundings. Conversely when the gas is compressed, the surroundings do work on the gas.
Internal Energy – Labelled![]() with a change in internal energy being labelled
with a change in internal energy being labelled![]() The internal energy is the energy of the particles of a system because of their random movement. It is in fact kinetic energy arising from the random motion of the particles of a system.
The internal energy is the energy of the particles of a system because of their random movement. It is in fact kinetic energy arising from the random motion of the particles of a system.
It is different from the energy of a system, because a system may have in general many kinds of energy – gravitational or elastic potential energy, chemical energy...
The internal energy of a system is in fact the heat energy, because heat is due to the random motion of the particles of a system. An increase in internal energy therefore means an increase in temperature, as does a change in phase from liquid to gas for example.
Ideal gas - An ideal gas is a gas that obeys the equation of state for an ideal gas
Mol - The mol is the basic SI unit for 'amount of substance'. One mol of a substance is the amount of that substance that contains the same number of particles as 12g of Carbon-12, ie
Avogadro's Constant - The number of particles in 1 mol, equal to
Molar Mass - The mass of 1 mol of a substance.
