Whenever a gas expands it does work on its' surroundings. If the pressure of the gas is changing, then calculating the work done can be complex. On the other hand, if the gas pressure is constant then finding the work done is especially simple.
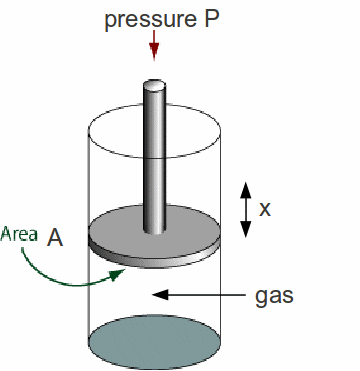
Suppose the piston is pushed back or upwards a distance![]() by the gas.
by the gas.
The pressure![]() pushing down on the piston exerts a force
pushing down on the piston exerts a force![]()
To force the piston back a distance x against a force![]() an amount of work equal to
an amount of work equal to![]() must be done by the gas.
must be done by the gas.![]() is the increase in volume
is the increase in volume![]() of the gas so we can write
of the gas so we can write![]() This equation is general and applies not only to pistons. It must be noted however in general an expanding gas cools down. The work supplied to expand the gas generally comes from the internal energy of the gas.
This equation is general and applies not only to pistons. It must be noted however in general an expanding gas cools down. The work supplied to expand the gas generally comes from the internal energy of the gas.
For example if a gas expands from![]() to
to![]() at atmospheric pressure, the work done is
at atmospheric pressure, the work done is![]()
