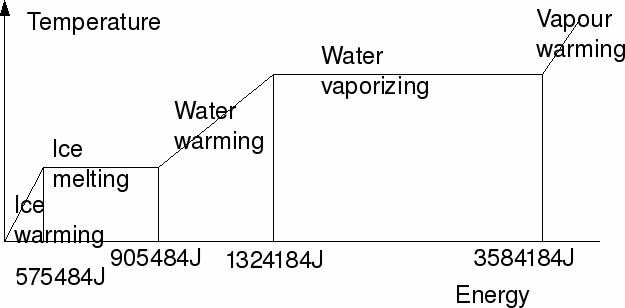
In general a substance may exist in more than one form – solid, liquid or gas.. To turn a substance from a solid to a liquid of a liquid to a gas needs energy. To heat a solid, liquid or gas also needs energy.
To heat m Kg ice by T degrees Celsius (or Kelvin): E=mcT
where c =specific heat capacity of ice =2108 J/Kg/K
To heat 1kg of ice from 0 degrees K to 273 degrees K takes 1*2108*273=575484J
To melt m Kg ice at 0 degrees Celsius (or 273 degrees Kelvin): E=ml
where l =specific latent heat of fusion of ice =330000 J/Kg
To melt 1Kg ice takes 1*330000=330000 J
To heat m Kg water by T degrees Celsius (or Kelvin): E=mcT
where c =specific heat capacity of water =4220 J/Kg/K
To heat 1Kg water by 100 degrees K takes 1*4220*100=422000 J
To vaporize m Kg water at 100 degrees Celsius (or 373 degrees Kelvin): E=ml
where l =specific latent heat of vaporization of water =2500000 J/Kg
To vaporize 1Kg water 1*250000J
To heat m Kg steam by T degrees Celsius (or Kelvin): E=mcT
where c=specific heat capacity of steam=1960 J/Kg/K
These are shown on the graph below.