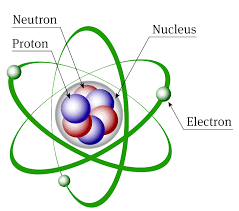
The Atomic Model
Atoms consist of a nucleus consisting of positively charged protons and neutral neutrons, surrounding by electrons moving in predefined orbits or shell.. The protons and neutrons together form the nucleons. Each nucleon has almost the same mass, and the mass of a nucleon is a convenient unit, so the mass of a nucleus consisting of 2 neutrons and 1 proton is taken to be 3.
The electron is very nearly massless, having only 1/1800 the mass of a nucleon.
