If a liquid is heated it's rate of evaporation will increase. If the liquid is being heated open to the atmosphere, it's saturated vapour pressure will increase. If it is heated further, at some point it's saturated vapour pressure will become equal to the atmospheric pressure. Further addition of heat will cause bubbles of vapour to form inside the body of the liquid and rise to the surface: the liquid boils.
The boiling point of a liquid is the temperature at which it's saturated vapour pressure is equal to the external atmospheric pressure.
The boiling point is not constant. Atmospheric pressure varies fom day to day, and also varies with the height above sea level. If atmospheric pressure increases, so must the saturated vapour pressure in order for the water to boil. His means that the liquid must be heated further and so the boiling point increases. The boiling point of water increases by 0.037 K if the pressure increases by 1 mm Hg for pressures close to atmospheric pressure (760 mm Hg).
A liquid can be made to boil at reduced pressure by reducing the atmospheric pressure above it. At the top of Mount Everest, the boiling point of water is only about 30 degrees Celsius. This principle is used inside a refrigerator pump - a liquid made to boil by reducing the pressure sucks the heat out of the inside of a refrigerator, cooling the contents.
The same principle is used in desalination plants. A vacuum pump is used to reduce the pressure above the water. In hot regions like the desert large areas can be covered with desalination tanks - the sun will heat the water in the tanks at reduced pressure. Vast quantities of fresh water can be produced - enough to supply cities. Desalination plants on ships can produce hundreds of tonnes of water each day, enough to supply the boilers and for all domestic uses.
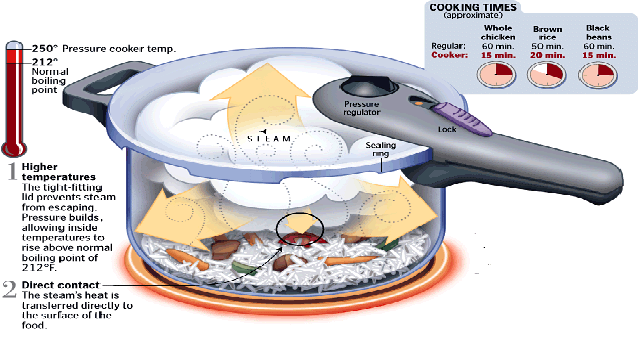
Moving the other way, increasing the temperature of the boiling point of water can reduce the cooking time for food, saving time and energy. This is the principle behind the pressure cooker.