Fire is a chemical reaction. In a fire, substances are being oxidised. Electrons are transferred atoms in the material that is being burnt to oxygen atoms. Oxygen from the air forms compounds with the combustible material, in the process taking electrons to form full, or at least more nearly full shells, which means a more stable molecule. In any flame therefore there will be ions – both positively and negatively charged – and this can be demonstrated by putting a burning candle in the presence of an electric field or connecting wires to the terminals of a battery and bringing the ends of the wire close to the flame.
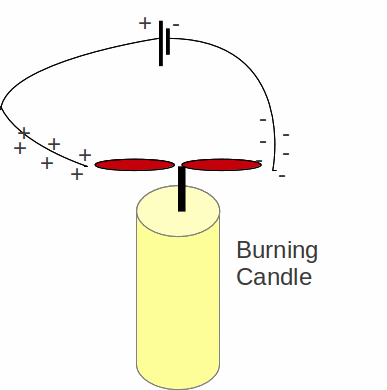
The candle flame is split and drawn in opposite directions. Positive ions in the field will be attracted to the wire connected to the negative terminal and negative ions in the flame will be attracted to the wire connected to the positive terminal.
