When a liquid evaporates, the molecules with the most energy evaporates first. The molecules with less energy, are left behind. Having less energy, the are at a lower temperature, since energy – specifically energy of random movement – is a measure of heat and temperature. We can use this mechanism to make ice by bubbling air through a beaker of ether. The beaker of ether is placed in a puddle of water.
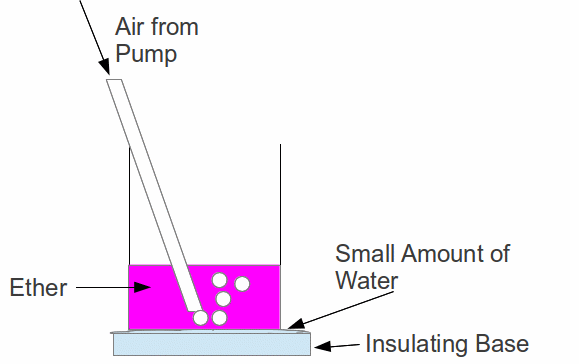
The ether evaporates into the bubbles. When the bubbles reach the surface the vapour escapes. The rapid change from liquid to vapour requires latent heat which comes from the internal (heat) energy of the ether, resulting in cooling. If the ether cools enough, it's temperature can fall below 0 ° C. Heat will be conducted through the base of the beaker from the small amount of water below it, and the water may turn to ice.
The same general principle – cooling by evaporation – is used in refrigerators.
