If a substance expands on solidifying then the application of pressure lowers the melting point. Conversely, substances which contract in volume on solidifying have their melting points raised by pressure. Thus the freezing point of water is lowered by 0.007K per atmosphere increase in pressure while the freezing point of paraffin increases by about 0.04K per atmosphere.
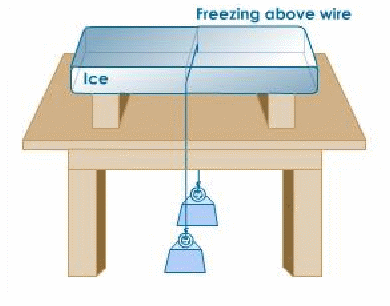
The diagram shows the effect of pressure on the melting point of ice in a practical way. Awire is connected between two weights and rests on a block of ice. After a while the wire has cut completely through the ice and rests on the table, leaving behind a solid block of ice. This phenomenon is called regelation. The pressure exerted by the wire lowers the melting point of ice in contact with it. The ice melts and water flows above the wire, then refreezes. Latent heat plays an important part. The wire gives out latent heat when it melts initially, which may be conducted through the wire to melt the ice below it. For this reason, copper for example, passes through the ice more quickly than iron, which is a worse conductor. A piece of string, which is a poor conductor, will not pass through at all.
Regelation assists in the making of snowballs. Compression of the snow in the hand causes some of it to melt, and on refreezing it binds the snow together. In very cold weather, the compression is insufficient to melt the snow, and snowballs are harder to make.
It also explains why ice is slippery. When you walk one ice, the pressure you exert lowers the melting point. It melts partially and the melted ice acts as a lubricant.
