The fundamental equation of quantum mechanics is the Schroedinger equation. It was introduced as a wave equation which did not contradict the relation between the energy and the momentum of a particle in classical mechanics. As a result, the wave function became a complex function. This looked quite strange, because using a complex number for a physical quantity could not be accepted. Then, what does the wave function mean?
As for the interpretation of the wave function,various ideas were proposed. Among them,the probability interpretation proposed by Born has been accepted to be the most orthodox, and quantum mechanics is constructed on the basis of this interpretation.
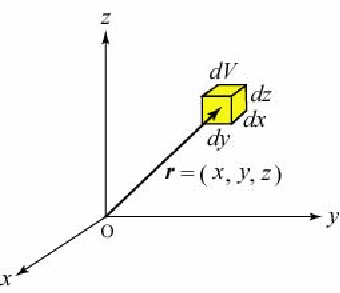
Suppose a small volume![]() with three edges of
with three edges of![]() and
and![]() at a coordinate point
at a coordinate point![]() in a three-dimensional space. Let the probability that a particle will be found in this volume at time
in a three-dimensional space. Let the probability that a particle will be found in this volume at time![]() be
be![]() The function
The function![]() is considered as the probability in the unit volume in the neighbourhood of the point
is considered as the probability in the unit volume in the neighbourhood of the point![]() and is sometimes called the probability density.
and is sometimes called the probability density.
This however does not mean that the particle e.g. an electron, exists in a form of a particle or corpuscle and this particle moves in accordance with the degree of probability. An electron is neither mere particle nor mere wave. An electron exists in both particle and wave states. The word probability used above denotes the chance that the particle will be found in the volume![]() when it is detected.
when it is detected.
Born's probability interpretation claims that the probability density that a particle will be found is equal to the square of the absolute value of the wave function. Namely, the probability that the particle will be found in the small volume![]() shown in the above figure is considered to be
shown in the above figure is considered to be ![]()
Although the wave function is a complex number in general, the square of the absolute value of it is always positive (or zero), and we have no difficulty.
