Apparatus:
Sealed round bottom flask with pressure gauge, water bath, bunsen burner, tripod, gauze, ice and thermometer.
Diagram:
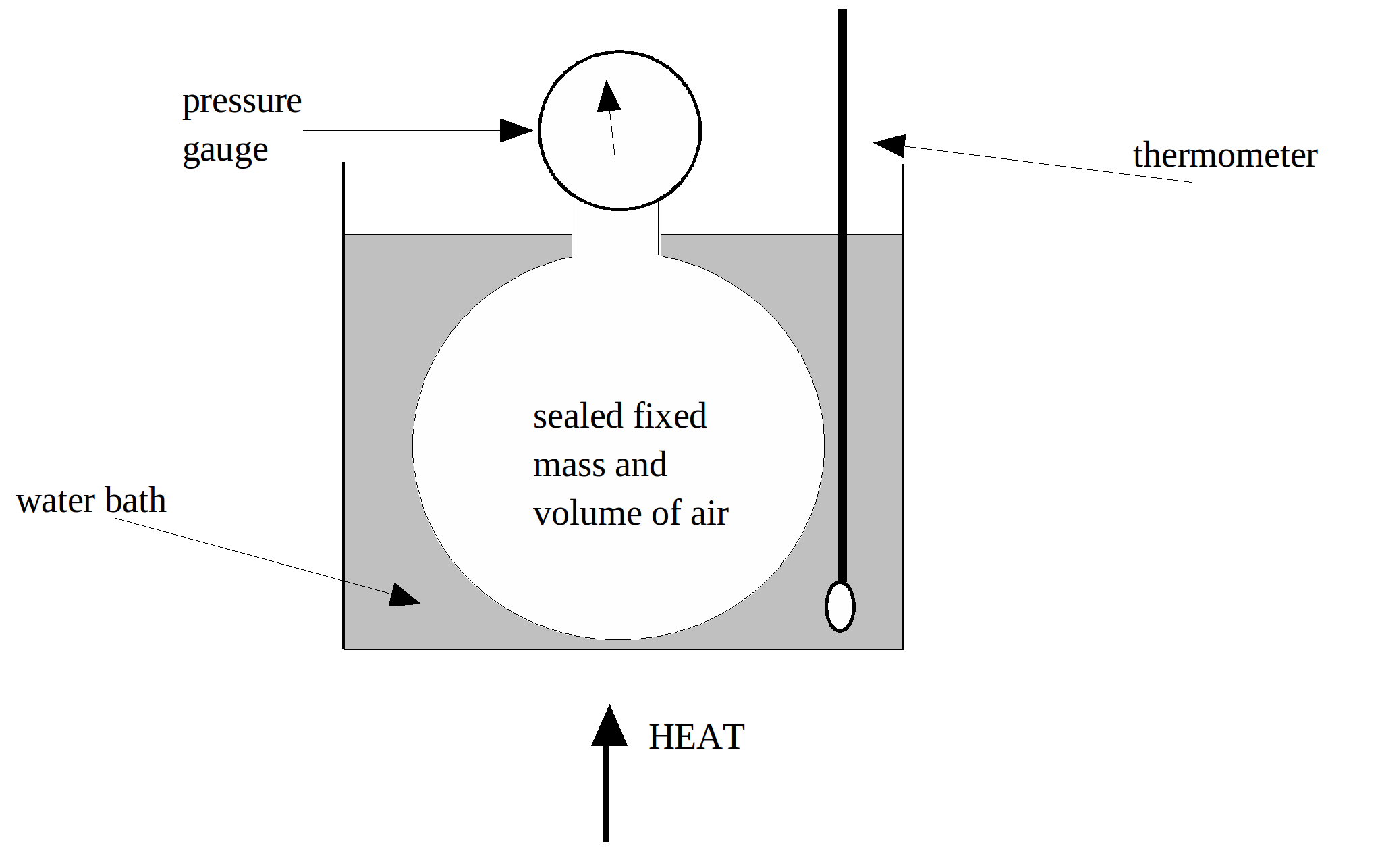
Procedure:
1. Place ice into the water bath and then insert the flask so that it is surrounded by the icy water.
2. Wait a few minutes and then note the temperature of the water bath (in oC) and the pressure p of the air in the flask. You will assume that the air temperature in the flask equals that of the water bath.
3. REMOVE ALL THE ICE FROM THE WATER BATH. Add water so that the water bath is about 4/5ths full when the flask is immersed in the water.
4. After a few minutes retake the temperature and pressure p readings.
5. Use a bunsen to heat the water bath and so obtain six more pressure![]() and temperature readings for up to a temperature of about 80oC. Do not go above a temperature of 80 Degrees C.
and temperature readings for up to a temperature of about 80oC. Do not go above a temperature of 80 Degrees C.
6. Plot a graph of pressure,![]() against temperature,
against temperature,![]()
7. Use your graph to gain an estimate of the value of absolute zero in Degrees C.
8. The pressure law states that for an ideal gas of constant mass and volume its pressure is proportional to its kelvin temperature.
Comment on whether or not your gas has obeyed the pressure law.
