Graphene is a one-atom-thick planar sheet of carbon atoms that are densely packed in a honeycomb crystal lattice. The name is derived from grahite because it was first made from graphite (by peeling of sheets of graphite with sellotape), and we may think of graphite as many sheets of graphene stacked on top of one another and graphene as atomic-scale chicken wire made of carbon atoms and their bonds.
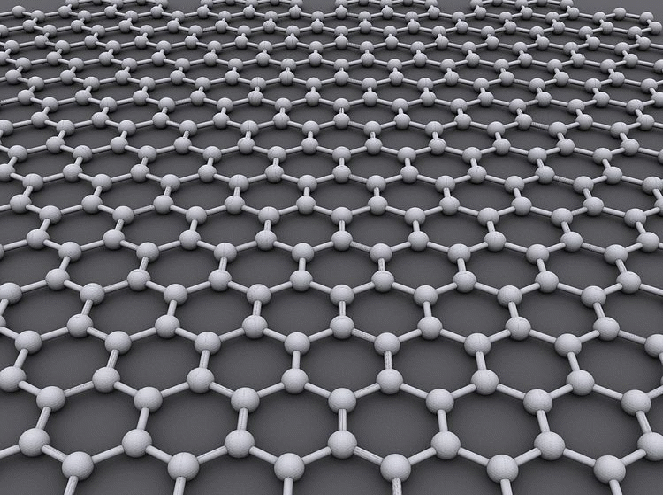
The carbon-carbon bond length in graphene is about 0.142 nm. Graphene sheets stack to form graphite with an interplanar spacing of 0.335 nm, which means that a stack of 3 million sheets would be only one millimeter thick. Graphene is the basic structural element of some carbon allotropes including graphite, charcoal, carbon nanotubes, and fullerenes. It can also be considered as an indefinitely large aromatic molecule, the limiting case of the family of flat polycyclic aromatic hydrocarbons.
Graphene is a semiconductor, and basic transistors have been made from it, and is amazingly strong – some 200 times stronger than steel.
