Alpha particle decay occurs when a helium nucleus consisting of two protons and two neutrons escapes from the nucleus. While inside the nucleus, the alpha particle is subject to the strong force, which is inherently much stronger than the repulsive electric force between the positively charged protons inside the nucleus.
It might be though therefore, that it is impossible for the alpha particle to escape the nucleus. This is obviously not true.
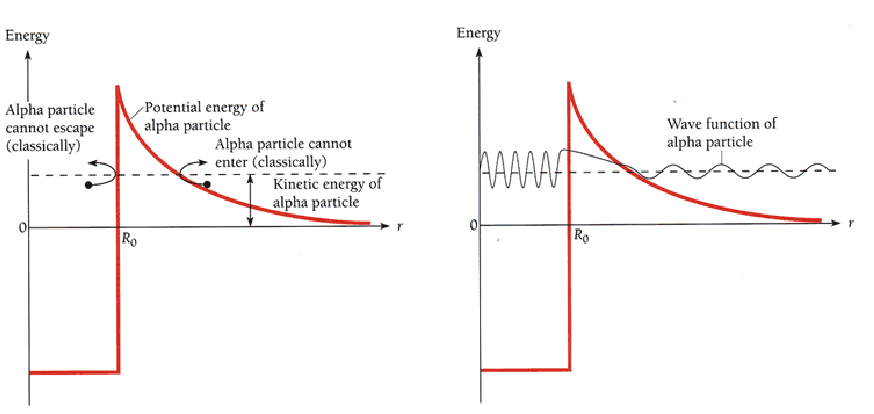 Representing the alpha particle classically, it has total energy equal to potential pluss kinetic energy, so if it has less energy than the maximum potential energy, it cannot escape the nucleus.
Representing the alpha particle classically, it has total energy equal to potential pluss kinetic energy, so if it has less energy than the maximum potential energy, it cannot escape the nucleus.
Quantum mechanics represents all particles as waves. The wave function representing the alpha particle penetrates into the into the potientiall wall, and even exits the other side. Since the wave function represents the probability of the particle being in there, a non zero value of the wavefunction at a point means a non zero probability of the particle being there.
You may picture the alpha particle as moving arround inside a thin walled well representing the nucleus. It hits the walls of the well many times a second, and there is a small possibility that when it hits the walls, it can escape. The probability depends on the difference between the energy of the alpha particle inside the well and the maximum potential energy of the well. The closer these are, the more likely the alpha particle is to escape each time it hits the wall of the well, and the shorter the half life.
