The density of ordinary matter is of the order of![]() but the density of the nucleus is much higher. Most of the volume of an atom is taken up by the cloud of electrons around the nucleus, the nucleus itself occupying a much smaller volume at the centre of the atom.
but the density of the nucleus is much higher. Most of the volume of an atom is taken up by the cloud of electrons around the nucleus, the nucleus itself occupying a much smaller volume at the centre of the atom.
The nuclear density in a typical nucleus can be approximately calculated. The radius of a typical nucleus is![]() where
where![]() is the mass number and
is the mass number and![]() is
is![]() The nuclear density
The nuclear density![]() satisfies
satisfies
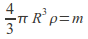 hence
hence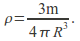
- The mass of
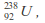 a uranium nucleus, is
a uranium nucleus, is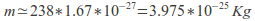 and
and 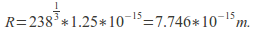 The density is then
The density is then -
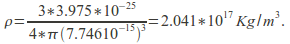
The components of an atom and of an atomic nucleus have varying densities. The proton is not a fundamental particle, being composed of quark-gluon matter. Its size is approximately![]() and its density
and its density![]() Using deep inelastic scattering, it has been estimated that the "size" of an electron, if it is not a point particle, must be less than
Using deep inelastic scattering, it has been estimated that the "size" of an electron, if it is not a point particle, must be less than![]() This would correspond to a density of roughly
This would correspond to a density of roughly![]()
Probing deeper within particles, one finds quarks which appear to be very dense and very hard. There are possibilities for still higher densities for quark matter, gluon matter, or neutrino matter.
Nuclear densities are not limited to the nucleus. A neutron star contains the mass of a star in a sphere only a few km in radius. The star consists of neutrons bound together in what is basically a giant atom, consisiting only of neutrons.
