Atomic nuclei consist of protons and neutrons bound together by the strong nuclear force, which is attractive. All the protons repel each other, all being positively charged. Neutrons, which are electrically neutral, stabilize the nucleus in two ways. They push the protons slightly apart, reducing the electrostatic repulsion, and they exert the attractive nuclear force on each other and on the protons. For this reason, one or more neutrons are necessary for two or more protons to be bound into a nucleus. As the number of protons increases, so does the ratio of neutrons to protons necessary to ensure a stable nucleus. A number of lighter elements have stable nuclides with a neutron to proton ratio 1:1 e.g.![]() and some even have more protons than neutrons e.g.
and some even have more protons than neutrons e.g.![]() but as the number of nucleons increases, nuclei need more proportionately more neutrons to remain stable.
but as the number of nucleons increases, nuclei need more proportionately more neutrons to remain stable.![]() is the heaviest stable nucleus with the same number of protons as neutrons.
is the heaviest stable nucleus with the same number of protons as neutrons.
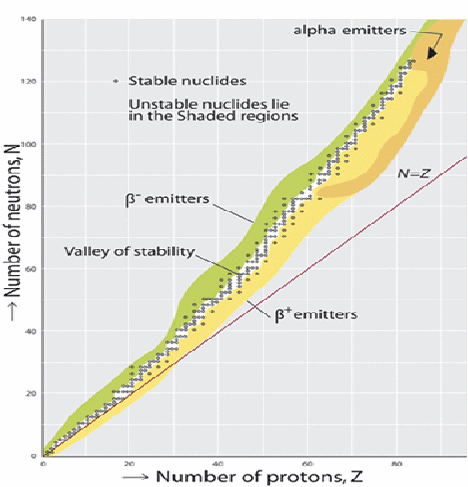
For heavier nucleons there is an excess of neutrons.
When nuclei decay, they tend to do so in such a way that the decay products move towards the zone of stability above.
If a nucleus emits an alpha particle, it lose two protons and two neutrons so moves left and down. If a nucleus emits a![]() (an electron), a neutron changes into a proton, so the nucleus moves right and down.
(an electron), a neutron changes into a proton, so the nucleus moves right and down.
If a nucleus emits a![]() (an positron), a proton changes into a neutron, so the nucleus moves left and up.
(an positron), a proton changes into a neutron, so the nucleus moves left and up.
