Any radioactive atom of a particular element with a certain number of protons and neutrons has a fixed and constant probability of decaying in a certain time period, usually taken as one second. The decay constant,![]() is a measure of this. We may write:
is a measure of this. We may write:
![]() (1)
(1)
The expression on the left hand side is the proportion of the N atoms at the start of the time period that decay in that time period.![]() is the probability that an individual atom decays in the that second and the negative sign means that
is the probability that an individual atom decays in the that second and the negative sign means that![]() is decreasing, or that atoms are decaying. We can show exponential decay in more than one way. We can show a graph of mass of undecayed substance – illustrated below, number of mols,
is decreasing, or that atoms are decaying. We can show exponential decay in more than one way. We can show a graph of mass of undecayed substance – illustrated below, number of mols,![]() (the activity), or N against t. All these graphs will show exponential decay.
(the activity), or N against t. All these graphs will show exponential decay.
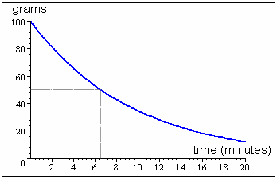
We can integrate (1) to find N in terms of t.
![]()
![]()
N in the equation above could be replaced by![]() (for mass),
(for mass),![]() (or activity),
(or activity),![]() (number of mols) and would still exhibit radioactive decay.
(number of mols) and would still exhibit radioactive decay.
Half Life
The half life of a radioactive substance is the time take for half of the material to decay. We can find an equation for the half life![]() in terms of
in terms of![]() When half the material has decayed,
When half the material has decayed,![]()
![]()
