When the temperature falls below 0°C, water freezes. The surface freezes first. Unlike most materials, for which the solid is denser than the liquid form, ice is less dense than water. Ice will not sink in water. Even more strange, as water is heated from 0°C, the water becomes denser up to a temeprature of 4°C, before becoming less dense with increasing temperature.
This means that when a lake freezes, the surface freezes first. Ice is an insulating material with a low coefficient of thermal conductivity, so the surface layer of ice acts to reduce further heat loss. Under the ice, though, the water will continue to slowly cool. The densest water will be at the bottom, and as long as the lake does not freeze solid, this means that the bottom of the lake will be at a temperature of 4°C. Transfer of heat energy by convection – the usual mode of heat transfer in fluids – ceases. The only mode of heat transfer is conduction, which is much less efficient at transferring heat.
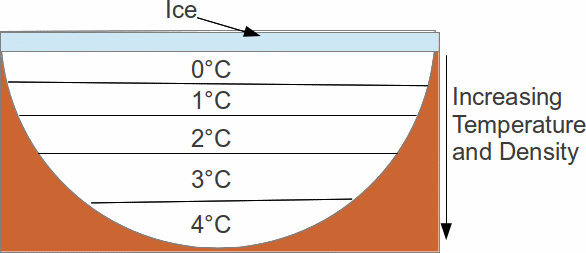
This means that it is virtually impossible for a lake to freeze solid. However cold it is, there will always be water at the bottom of a frozen lake, at a temperature of 4°C where life vcan survive.
